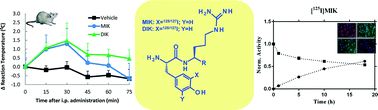
Abstract
Amidated kyotorphin (L-Tyr-L-Arg-NH2; KTP-NH2) shows analgesic properties following systemic administration. Although KTP-NH2 does not have toxic effects in the liver, its biodistribution is unknown. KTP-NH2 was radioiodinated to evaluate its biological fate in vivo. Mono-radioiodinated KTP-NH2 ([125I]MIK) was radiochemically stable in vitro, namely in saline at 4 °C up to 1 week, and moderately stable when incubated with human serum at 37 °C. Although the radioiodinated peptide could translocate a cellular model of the blood–brain barrier (BBB), the levels of radioactivity in the brain were minimal, 5 and 10 min after intraperitoneal injection (i.p.). Significant accumulation of 125I was found in the thyroid probably reflecting the hydrolysis of the iodine–tyrosine bond by liver deiodinases. HPLC analysis of plasma samples collected 5 min post-injection showed that intact [125I]MIK accounted for 72.5% of the total radioactivity, whereas the remaining radioactivity was associated with free radioiodide (I−). These findings were subsequently confirmed by the low radioactivity found in the liver and kidney homogenates from rats sacrificed 10 min after i.p. administration. The analgesic activity of mono- and di-iodinated KTP-NH2 was also evaluated by a hot plate assay showing a delayed peak of maximal efficacy compared to KTP-NH2 (30 vs. 15 minutes). Overall, the peripheral effects of the peptides cannot be excluded.

 Please wait while we load your content...
Please wait while we load your content...