Methods of protein surface PEGylation under structure preservation for the emulsion-based formation of stable nanoparticles†‡
Abstract
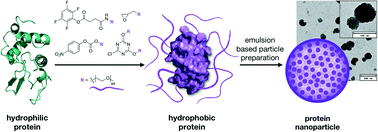
Proteins show remarkable versatility as multifunctional materials for therapeutic applications. They can be easily modified with the toolkit of bioorganic chemistry and are particularly attractive because of their degradability and biocompatibility. Herein, we evaluate different methods for the attachment of multiple PEG chains on the surface of the enzyme lysozyme. For this, we activated standard 2 kDa mPEG chains with four different electrophilic groups and tested their ability to react with different amino acids on the surface of our model protein. The aim was to find an effective and at the same time mild modification method that preserves the native structure and activity of the enzyme. The amphiphilic properties of PEG induce a solubility switch of the protein material which allows the formation of nanoparticles using a nano-emulsion technique in the size range of 100–130 nm. We found that, even though all produced materials are soluble in organic solvents, the amount of introduced PEG chains and the enzyme activity significantly vary depending on the chosen PEGylation method.

 Please wait while we load your content...
Please wait while we load your content...