Characterization of substrate binding and enzymatic removal of a 3-methyladenine lesion from genomic DNA with TAG of MDR A. baumannii†
Abstract
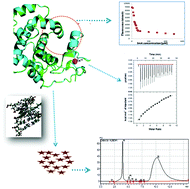
The rise of multiple-drug resistance in bacterial pathogens imposes a serious public health concern and has led to increased interest in studying various pathways as well as enzymes. Different DNA glycosylases collaborate during bacterial infection and disease by overcoming the effects of ROS- and RNS-mediated host innate immunity response. 3-Methyladenine DNA glycosylase I, an essential DNA repair enzyme, was chosen for the present study from the MDR species of A. baumannii. The enzyme was especially chosen because of its functional significance in A. baumannii and due to its structural variation from its human homologue. MDR strains such as A. baumannii are interesting targets owing to their evolved mechanisms of evading a host defence. In the absence of any structural information, the enzyme was characterized biophysically and biochemically. Binding studies with 3mA and Zn2+ indicated that the activity of TAG-Ab is an enthalpy-driven process. Fluorescence thermal denaturation studies described that the denaturation of TAG-Ab is a two-step process. Modified RP-HPLC-based glycosylase assay attested that the heterologously expressed and purified TAG-Ab enzyme is active and catalyses the removal of 3mA. Other binding parameters and the effect of adenine on substrate binding are also discussed in detail.
 Please wait while we load your content...
Please wait while we load your content...