Transient microfluidic compartmentalization using actionable microfilaments for biochemical assays, cell culture and organs-on-chip†
Abstract
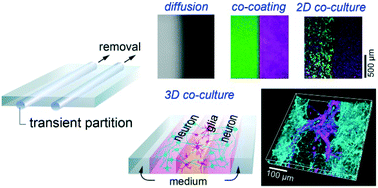
We report here a simple yet robust transient compartmentalization system for microfluidic platforms. Cylindrical microfilaments made of commercially available fishing lines are embedded in a microfluidic chamber and employed as removable walls, dividing the chamber into several compartments. These partitions allow tight sealing for hours, and can be removed at any time by longitudinal sliding with minimal hydrodynamic perturbation. This allows the easy implementation of various functions, previously impossible or requiring more complex instrumentation. In this study, we demonstrate the applications of our strategy, firstly to trigger chemical diffusion, then to make surface co-coating or cell co-culture on a two-dimensional substrate, and finally to form multiple cell-laden hydrogel compartments for three-dimensional cell co-culture in a microfluidic device. This technology provides easy and low-cost solutions, without the use of pneumatic valves or external equipment, for constructing well-controlled microenvironments for biochemical and cellular assays.


 Please wait while we load your content...
Please wait while we load your content...