Brain-on-a-chip model enables analysis of human neuronal differentiation and chemotaxis†
Abstract
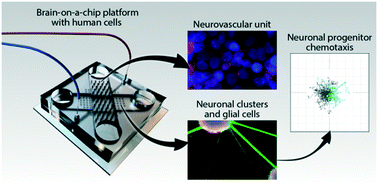
Migration of neural progenitors in the complex tissue environment of the central nervous system is not well understood. Progress in this area has the potential to drive breakthroughs in neuroregenerative therapies, brain cancer treatments, and neurodevelopmental studies. To a large extent, advances have been limited due to a lack of controlled environments recapitulating characteristics of the central nervous system milieu. Reductionist cell culture models are frequently too simplistic, and physiologically more relevant approaches such as ex vivo brain slices or in situ experiments provide little control and make information extraction difficult. Here, we present a brain-on-chip model that bridges the gap between cell culture and ex vivo/in vivo conditions through recapitulation of self-organized neural differentiation. We use a new multi-layer silicone elastomer device, over the course of four weeks to differentiate pluripotent human (NTERA2) cells into neuronal clusters interconnected with thick axonal bundles and interspersed with astrocytes, resembling the brain parenchyma. Neurons within the device express the neurofilament heavy (NF200) mature axonal marker and the microtubule-associated protein (MAP2ab) mature dendritic marker, demonstrating that the devices are sufficiently biocompatible to allow neuronal maturation. This neuronal-glial environment is interfaced with a layer of human brain microvascular endothelial cells showing characteristics of the blood–brain barrier including the expression of zonula occludens (ZO1) tight junctions and increased trans-endothelial electrical resistance. We used this device to model migration of human neural progenitors in response to chemotactic cues within a brain-tissue setting. We show that in the presence of an environment mimicking brain conditions, neural progenitor cells show a significantly enhanced chemotactic response towards shallow gradients of CXCL12, a key chemokine expressed during embryonic brain development and in pathological tissue regions of the central nervous system. Our brain-on-chip model thus provides a convenient and scalable model of neural differentiation and maturation extensible to analysis of complex cell and tissue behaviors.

 Please wait while we load your content...
Please wait while we load your content...