5-Hydroxymethylcytosine in E-box motifs ACAT|GTG and ACAC|GTG increases DNA-binding of the B-HLH transcription factor TCF4†
Abstract
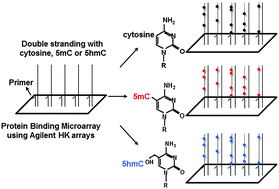
We evaluated DNA binding of the B-HLH family members TCF4 and USF1 using protein binding microarrays (PBMs) containing double-stranded DNA probes with cytosine on both strands or 5-methylcytosine (5mC) or 5-hydroxymethylcytosine (5hmC) on one DNA strand and cytosine on the second strand. TCF4 preferentially bound the E-box motif (CAN|NTG) with strongest binding to the 8-mer CAG|GTGGT. 5mC uniformly decreases DNA binding of both TCF4 and USF1. The bulkier 5hmC also inhibited USF1 binding to DNA. In contrast, 5hmC dramatically enhanced TCF4 binding to E-box motifs ACAT|GTG and ACAC|GTG, being better bound than any 8-mer containing cytosine. Examination of X-ray structures of the closely related TCF3 and USF1 bound to DNA suggests TCF3 can undergo a conformational shift to preferentially bind to 5hmC while the USF1 basic region is bulkier and rigid precluding a conformation shift to bind 5hmC. These results greatly expand the regulatory DNA sequence landscape bound by TCF4.

 Please wait while we load your content...
Please wait while we load your content...