Improving the efficiency of the Diels–Alder process by using flow chemistry and zeolite catalysis†
Abstract
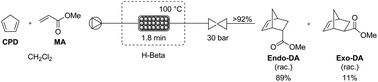
The industrial application of the Diels–Alder reaction for the atom-efficient synthesis of (hetero)cyclic compounds constitutes an important challenge. Safety and purity concerns, related to the instability of the polymerization prone diene and/or dienophile, limit the scalability of the production capacity of Diels–Alder products in a batch mode. To tackle these problems, the use of a high-pressure continuous microreactor process was considered. In order to increase the yields and the selectivity towards the endo-isomer, commercially available zeolites were used as a heterogeneous catalyst in a microscale packed bed reactor. As a result, a high conversion (≥95%) and endo-selectivity (89 : 11) were reached for the reaction of cyclopentadiene and methyl acrylate, using a 1 : 1 stoichiometry. A throughput of 0.87 g h−1 during at least 7 h was reached, corresponding to a 3.5 times higher catalytic productivity and a 14 times higher production of Diels–Alder adducts in comparison to the heterogeneous lab-scale batch process. Catalyst deactivation was hardly observed within this time frame. Moreover, complete regeneration of the zeolite was demonstrated using a straightforward calcination procedure.


 Please wait while we load your content...
Please wait while we load your content...