Clean synthesis of primary to tertiary carboxamides by CsOH-catalyzed aminolysis of nitriles in water†
Abstract
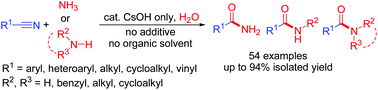
Using CsOH as the only catalyst and utilizing its “cesium effect”, a clean synthesis of a wide range of primary, secondary, and tertiary carboxamides was achieved by aminolysis reactions of nitriles with ammonia, primary, or secondary amines in water. Studies on the control reactions revealed that the reactions with ammonia most probably proceed via an aminolysis path by the initial addition of ammonia to Cs-activated nitriles to form unsubstituted amidine intermediates, while the reactions with primary or secondary amines may proceed via a hydration/transamidation path by the initial hydration of the Cs-activated nitriles to form primary carboxamide intermediates followed by their transamidation with amines through the formation of substituted amidine intermediates.

 Please wait while we load your content...
Please wait while we load your content...