Maleic acid and aluminum chloride catalyzed conversion of glucose to 5-(hydroxymethyl) furfural and levulinic acid in aqueous media†
Abstract
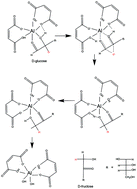
Maleic acid (MA) and AlCl3 self-assemble into catalytic complexes (Al–(MA)2–(OH)2(aq)) with improved selectivity for converting glucose to HMF, and levulinic acid. The calculated activation energy (Ea) of the MA–aluminum catalyzed glucose-to-fructose isomerization is 95 kJ mol−1 compared to 149 kJ mol−1 for HCl and AlCl3 alone. Furthermore, conversion of fructose to HMF is enhanced. The catalytic conversion of fructose to HMF by MA and AlCl3 at 180 °C is 1.7× faster with 3.3× higher selectivity when compared to HCl with AlCl3. Liquid 13C NMR spectra results indicate that glucose undergoes a ring-opening process in aqueous solution when maleic acid is introduced, which we hypothesize facilitates the hydride shift in glucose for isomerization leading to enhanced rates and selectivity. Improved selectivity of glucose conversion to HMF and levulinic acid could improve the economics of producing these value-added chemicals for use in renewable, sustainable polymers.


 Please wait while we load your content...
Please wait while we load your content...