Chemical cascades in water for the synthesis of functionalized aromatics from furfurals†
Abstract
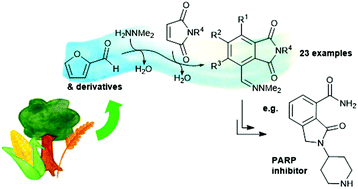
One-pot synthetic routes from furfurals to polysubstituted aromatic compounds have been developed in water, without the need for any organic solvents. The reaction proceeds via an uncatalysed, one-pot reaction cascade through formation of a hydrazone derivative, in situ cycloaddition with a dienophile, then aromatisation. A range of substituted phthalimides can be accessed with complete control over the substitution pattern. The reaction was also extended to other dienophiles and the diene 2-furylacrolein. The phthalimide products were further elaborated to produce a variety of polysubstituted benzenes including pharmaceutically relevant compounds.

 Please wait while we load your content...
Please wait while we load your content...