Identifying thermal phase transitions of lignin–solvent mixtures using electrochemical impedance spectroscopy†
Abstract
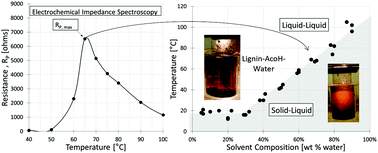
Lignin is unique among renewable biopolymers in having significant aromatic character, making it potentially attractive for a wide range of uses from coatings to carbon fibers. Recent research has shown that hot acetic acid (AcOH)–water mixtures can be used to recover “ultraclean” lignins of controlled molecular weight from Kraft lignins. A key feature of this discovery is the existence of a region of liquid–liquid equilibrium (LLE), with one phase being rich in the purified lignin and the other rich in solvent. Although visual methods can be used to determine the temperature at which solid lignin melts in the presence of AcOH–water mixtures to form LLE, the phase transition can be seen only at lower AcOH concentrations due to solvent opacity. Thus, an electrochemical impedance spectroscopy (EIS) technique was developed for measuring the phase-transition temperature of a softwood Kraft lignin in AcOH–water mixtures. In electrochemical cells, the resistance to double-layer charging (i.e., polarization resistance Rp) is related to the concentration and mobility of free ions in the electrolyte, both of which are affected by the phases present. When the lignin–AcOH–water mixture was heated through the phase transition, RP was found to be a strong function of temperature, with the maximum in RP corresponding to the transition temperature obtained from visual observation. As the system is heated, acetate ions associate with the solid lignin, forming a liquefied, lignin-rich phase. This association increases the overall impedance of the system, as mobile acetate ions are stripped from the solvent phase and thus are no longer available to adsorb on the polarizing electrode surfaces. The maximum in RP occurs once the new lignin-rich phase has completely formed, and no further association of the lignin polymer with AcOH is possible. Except at sub-ambient temperatures, the phase-transition temperature was a strong function of solvent composition, increasing linearly from 18 °C at 70/30 AcOH/water to 97 °C at 10/90 wt% AcOH/water.


 Please wait while we load your content...
Please wait while we load your content...