Sustainable synthesis of enantiopure fluorolactam derivatives by a selective direct fluorination – amidase strategy†
Abstract
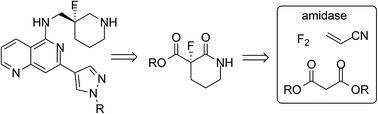
Pharmaceutically important chiral fluorolactam derivatives bearing a fluorine atom at a stereogenic centre were synthesized by a route involving copper catalyzed selective direct fluorination using fluorine gas for the construction of the key C–F bond and a biochemical amidase process for the crucial asymmetric cyclisation stage. A comparison of process green metrics with reported palladium catalyzed enantioselective fluorination methodology shows the fluorination-amidase route to be very efficient and more suitable for scale-up.

 Please wait while we load your content...
Please wait while we load your content...