Probing the mechanism of benzaldehyde reduction to chiral hydrobenzoin on the CNT surface under near-UV light irradiation†
Abstract
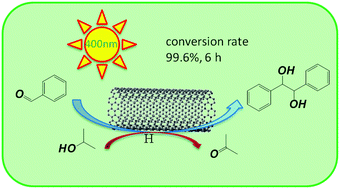
Metal-free CNTs exhibit high activity (conversion rate 99.6%, 6 h) towards the synthesis of chiral hydrobenzoin from benzaldehyde under near-UV light irradiation (320–400 nm). The CNT structure before and after the reaction, the interaction between the molecule and the CNT surface, the intermediate products, the substitution effect and the influence of light on the reaction were examined using various techniques. A photo-excited conduction electron transfer (PECET) mechanism for the photocatalytic reduction using CNTs has been proposed. This finding provides a green photocatalytic route for the production of hydrobenzoin and highlights a potential photocatalytic application of CNTs.

 Please wait while we load your content...
Please wait while we load your content...