Statistical generation of training sets for measuring NO3−, NH4+ and major ions in natural waters using an ion selective electrode array†
Abstract
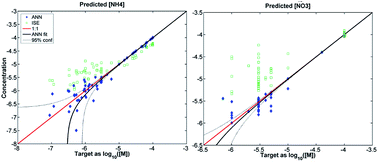
Knowledge of ionic concentrations in natural waters is essential to understand watershed processes. Inorganic nitrogen, in the form of nitrate and ammonium ions, is a key nutrient as well as a participant in redox, acid–base, and photochemical processes of natural waters, leading to spatiotemporal patterns of ion concentrations at scales as small as meters or hours. Current options for measurement in situ are costly, relying primarily on instruments adapted from laboratory methods (e.g., colorimetric, UV absorption); free-standing and inexpensive ISE sensors for NO3− and NH4+ could be attractive alternatives if interferences from other constituents were overcome. Multi-sensor arrays, coupled with appropriate non-linear signal processing, offer promise in this capacity but have not yet successfully achieved signal separation for NO3− and NH4+in situ at naturally occurring levels in unprocessed water samples. A novel signal processor, underpinned by an appropriate sensor array, is proposed that overcomes previous limitations by explicitly integrating basic chemical constraints (e.g., charge balance). This work further presents a rationalized process for the development of such in situ instrumentation for NO3− and NH4+, including a statistical-modeling strategy for instrument design, training/calibration, and validation. Statistical analysis reveals that historical concentrations of major ionic constituents in natural waters across New England strongly covary and are multi-modal. This informs the design of a statistically appropriate training set, suggesting that the strong covariance of constituents across environmental samples can be exploited through appropriate signal processing mechanisms to further improve estimates of minor constituents. Two artificial neural network architectures, one expanded to incorporate knowledge of basic chemical constraints, were tested to process outputs of a multi-sensor array, trained using datasets of varying degrees of statistical representativeness to natural water samples. The accuracy of ANN results improves monotonically with the statistical representativeness of the training set (error decreases by ∼5×), while the expanded neural network architecture contributes a further factor of 2–3.5 decrease in error when trained with the most representative sample set. Results using the most statistically accurate set of training samples (which retain environmentally relevant ion concentrations but avoid the potential interference of humic acids) demonstrated accurate, unbiased quantification of nitrate and ammonium at natural environmental levels (±20% down to <10 μM), as well as the major ions Na+, K+, Ca2+, Mg2+, Cl−, and SO42−, in unprocessed samples. These results show promise for the development of new in situ instrumentation for the support of scientific field work.

 Please wait while we load your content...
Please wait while we load your content...