Nitrogenase bioelectrocatalysis: heterogeneous ammonia and hydrogen production by MoFe protein†
Abstract
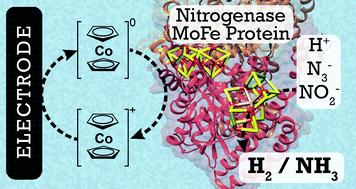
Nitrogenase is the only enzyme known to catalyze the reduction of N2 to 2NH3. In vivo, the MoFe protein component of nitrogenase is exclusively reduced by the ATP-hydrolyzing Fe protein in a series of transient association/dissociation steps that are linked to the hydrolysis of two ATP for each electron transferred. We report MoFe protein immobilized at an electrode surface, where cobaltocene (as an electron mediator that can be observed in real time at a carbon electrode) is used to reduce the MoFe protein (independent of the Fe protein and of ATP hydrolysis) and support the bioelectrocatalytic reduction of protons to dihydrogen, azide to ammonia, and nitrite to ammonia. Bulk bioelectrosynthetic N3− or NO2− reduction (50 mM) for 30 minutes yielded 70 ± 9 nmol NH3 and 234 ± 62 nmol NH3, with NO2− reduction operating at high faradaic efficiency.

 Please wait while we load your content...
Please wait while we load your content...