First demonstration of direct hydrocarbon fuel production from water and carbon dioxide by solar-driven thermochemical cycles using rhodium–ceria†
Abstract
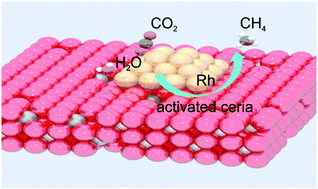
Solar-driven thermochemical cycles (STCs) are one of the direct pathways to store solar energy in the chemical bonds of energy-rich molecules. By utilizing a redox material like ceria (CeO2) as reactive medium, STCs can produce the chemical fuels hydrogen and carbon monoxide from water and carbon dioxide. The produced syngas, a mixture of hydrogen and carbon monoxide, can be upgraded to hydrocarbon fuels by the Fischer–Tropsch process. Here, we explore a new concept of producing hydrocarbon fuels directly from water and carbon dioxide by incorporating a catalytic process into STCs. To achieve this, a starting material of ceria doped with a catalyst is used as reactive medium. The primary role of the catalyst is to catalyze the formation of hydrocarbon molecules during the reoxidation of ceria by water and carbon dioxide. In this study, nickel-doped ceria and rhodium-doped ceria were investigated for their methane formation activity after being activated by chemical or thermal reduction. Both materials, after being reduced by hydrogen at 600 °C, are active in producing methane during their reoxidation by water and carbon dioxide at 500 °C. After being thermally reduced at extreme temperatures of 1400 °C and 1500 °C, metallic rhodium is formed in rhodium-doped ceria. The activated rhodium–ceria produces methane directly from water and carbon dioxide during reoxidation. The long-term methane formation activity of rhodium–ceria for 59 cycles with thermal reduction is reported. With rhodium–ceria, this study demonstrates for the first time the concept of producing hydrocarbon fuels, i.e. methane, directly from water and carbon dioxide by realistic STCs. In contrast, nickel-doped ceria is not active in producing methane after thermal activation, owing to rapid sintering and loss of nickel at high temperatures. This underlines the importance of evaluating the effect of thermal reduction on the redox material used. The material's physicochemical properties could be rapidly and significantly altered at the extreme temperatures required for the thermal reduction of ceria. Such changes may render a material that is active and stable at low temperatures inactive when used under realistic conditions of STCs.

 Please wait while we load your content...
Please wait while we load your content...