Hexamethyl-p-terphenyl poly(benzimidazolium): a universal hydroxide-conducting polymer for energy conversion devices†
Abstract
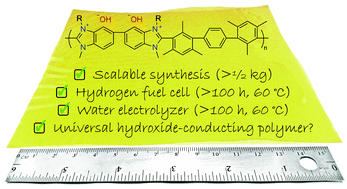
A hydroxide-conducting polymer, HMT-PMBI, which is prepared by methylation of poly[2,2′-(2,2′′,4,4′′,6,6′′-hexamethyl-p-terphenyl-3,3′′-diyl)-5,5′-bibenzimidazole] (HMT-PBI), is utilized as both the polymer electrolyte membrane and ionomer in an alkaline anion-exchange membrane fuel cell and alkaline polymer electrolyzer. A fuel cell operating between 60 and 90 °C and subjected to operational shutdown, restarts, and CO2-containing air demonstrates remarkable in situ stability for >4 days, over which its performance improved. An HMT-PMBI-based fuel cell was operated at current densities >1000 mA cm−2 and power densities of 370 mW cm−2 at 60 °C. When similarly operated in a water electrolyzer with circulating 1 M KOH electrolyte at 60 °C, its performance was unchanged after 8 days of operation. Methodology for up-scaled synthesis of HMT-PMBI is presented, wherein >½ kg is synthesized in six steps with a yield of 42%. Each step is optimized to achieve high batch-to-batch reproducibility. Water uptake, dimensional swelling, and ionic conductivity of HMT-PMBI membranes exchanged with various anions are reported. In the fully-hydrated chloride form, HMT-PMBI membranes are mechanically strong, and possess a tensile strength and Young's modulus of 33 MPa and 225 MPa, respectively, which are significantly higher than Nafion 212, for example. The hydroxide anion form shows remarkable ex situ chemical and mechanical stability and is seemingly unchanged after a 7 days exposure to 1 M NaOH at 80 °C or 6 M NaOH at 25 °C. Only 6% chemical degradation is observed when exposed to 2 M NaOH at 80 °C for 7 days. The ease of synthesis, synthetic reproducibility, scale-up, and exceptional in situ and ex situ properties of HMT-PMBI renders this a potential benchmark polymer for energy conversion devices requiring an anion-exchange material.

 Please wait while we load your content...
Please wait while we load your content...