Regulating the anticancer properties of organometallic dendrimers using pyridylferrocene entities: synthesis, cytotoxicity and DNA binding studies†
Abstract
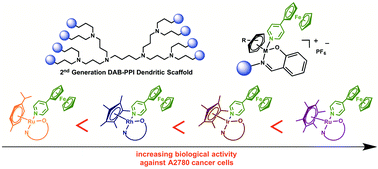
A new series of eight first- and second-generation heterometallic ferrocenyl-derived metal–arene metallodendrimers, containing ruthenium(II)–p-cymene, ruthenium(II)–hexamethylbenzene, rhodium(III)–cyclopentadienyl or iridium(III)–cyclopentadienyl moieties have been prepared. The metallodendrimers were synthesized by first reacting DAB-(NH2)n (where n = 4 or 8, DAB = diaminobutane) with salicylaldehyde, and then the Schiff-base dendritic ligands were reacted in a one-pot reaction with the appropriate [(η6-p-iPrC6H4Me)RuCl2]2, [(η6-C6Me6)RuCl2]2, [(η5-C5Me5)IrCl2]2 or [(η5-C5Me5)RhCl2]2 dimers, in the presence of 4-pyridylferrocene. Heterometallic binuclear analogues were prepared as models of the larger metallodendrimers. All complexes have been characterized using analytical and spectroscopic methods. The cytotoxicity of the heterometallic metallodendrimers and their binuclear analogues were evaluated against A2780 cisplatin-sensitive and A2780cisR cisplatin-resistant human ovarian cancer cell lines and against a non-tumorigenic HEK-293 human embryonic kidney cell line. The second generation Ru(II)-η6-C6Me6 metallodendrimer is the most cytotoxic and selective compound. DNA binding experiments reveal that a possible mode-of-action of these compounds involves non-covalent interactions with DNA.

 Please wait while we load your content...
Please wait while we load your content...