The molecular mechanism of palladium-catalysed cyanoesterification of methyl cyanoformate onto norbornene†
Abstract
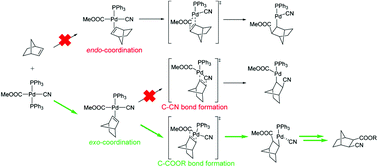
Potential energy surface mapping was completed for the entire catalytic cycle of palladium-catalysed cyanoesterification onto norbornene (NBE) using density functional theory calculations. We found that after the oxidative addition step of the reagent methyl cyanoformate, the reaction proceeds through an insertion of olefin into a PdII–COOMe bond first. Subsequently, reductive elimination occurs by transferring the cyanide group from the Pd center to NBE. This rearrangement is triggered by the rotation of the ester group into a π-interaction with the PdII centre. The regioselectivity of olefin insertion is controlled by ionic and covalent interactions in the precursor π-complex formation step. Importantly, all of the intermediates and transition states along the exo pathway were found to be more stable than the corresponding structures of the endo pathway without any sign of crossing over between the two surfaces via isomerization. The rate-determining step is the reductive elimination despite the fact that the corresponding activation barrier is reduced by conformational changes via the rotation of a MeOOC–C(NBE) bond.

 Please wait while we load your content...
Please wait while we load your content...