Anion binding, electrochemistry and solvatochromism of β-brominated oxoporphyrinogens†
Abstract
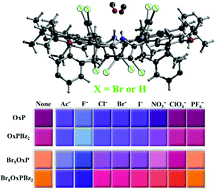
Effects of macrocycle bromination on the structural, electrochemical and anion binding properties of 5,10,15,20-tetrakis(3,5-di-t-butyl-4-oxo-cyclohexa-2,5-dienylidene)porphyrinogen, OxP, are reported. Bromination of 5,10,15,20-tetrakis(3,5-di-t-butyl-4-hydroxyphenyl)-porphinatocopper(II), [T(DtBHP)P]Cu(II) yielded β-Br8OxP, which was N-alkylated to β-Br8OxPBz2 and β-Br8OxPBz4 (where Bz = 4-bromobenzyl). β-Br8OxPBz2 crystallizes in orthorhombic space group Pccn [a = 23.5535(17) Å, b = 19.3587(14) Å c = 20.9760(15) Å, V = 9564.3(12) Å3]. It has a calix[4]pyrrole-like structure with a saddle conformation and two molecules of methanol occupy a central binding site made up of the non-alkylated pyrrole N–H groups. Computational and electrochemical studies revealed widening HOMO–LUMO band gaps for the brominated compounds over the non-brominated analogues consistent with the observed hypsochromic shifts in electronic absorption spectra. Solvatochromic and chromogenic effects on anion binding were both observed for β-Br8OxP and β-Br8OxPBz2 with binding affinities of anions being greater than those observed for the corresponding OxP and OxPBz2. Colorimetric sensor studies suggest that the OxP compounds reported here are possible candidates for use in the design of optoelectronic noses for detection of anions and anionic analyte species of biological interest.

 Please wait while we load your content...
Please wait while we load your content...