Halochromic coordination polymers based on a triarylmethane dye for reversible detection of acids†
Abstract
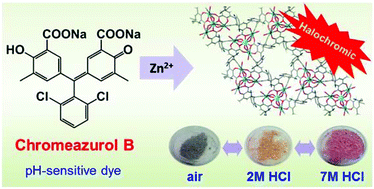
Chromeazurol B (Na2HL) is a pH-sensitive (halochromic) dye based on a hydroxytriarylmethane core and two carboxylate functional groups, which makes it suitable for the synthesis of coordination polymers. Two new coordination polymers [NaZn4(H2O)3(L)3]·3THF·3H2O (1) and [Zn3(H2O)3(μ2-OH2)(μ3-OH)(HL)2(H2L)]·2THF·3H2O (2) incorporating Chromeazurol B linkers have been prepared and characterised. The structure of 1 comprises pentanuclear heterometallic {Zn4Na} nodes linked by six L3− anions to give a layered structure with a honeycomb topology. 2 crystallizes as a double-chain ribbon (ladder) structure with two types of metal node: a mononuclear Zn(II) cation and tetranuclear {Zn(II)}4 cluster. Chromeazurol B anions link each tetranuclear cluster to four individual Zn(II) cations and each Zn(II) cation with four tetranuclear clusters. Both compounds show pH-sensitivity in water solution which can be observed visually, giving the first example of a halochromic coordination polymer. The halochromic properties of 1 towards HCl vapors were systematically investigated. As-synthesized violet-grey 1 reversibly changes color from orange to pink in the presence of vapors of 2 M and 7 M HCl, respectively. The coordination of the Chromeazurol B anion at each color stage was examined by diffuse reflectance spectroscopy and FT-IR measurements. The remarkable stability of 1 to acid and the observed reversible and reproducible color changes provide a new design for multifunctional sensor materials.


 Please wait while we load your content...
Please wait while we load your content...