Preparation and sensing properties of a nitrogen-rich ferrocene-imidazole-quinoxaline triad decorated with pyrrole rings†
Abstract
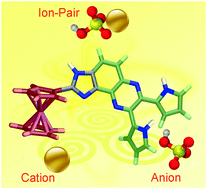
The synthesis and sensing properties of the nitrogen-rich ferrocene-imidazole-quinoxaline triad 1 decorated with two pyrrole rings have been described. Due to its ditopic nature, this molecule behaves as an ion-pair receptor for Ni2+cations and AcO− anions, although no affinity for either of the discrete ions is observed. It also displays the rare property consistent with the cooperative AND recognition of ion pairs. Thus, this receptor shows an important enhancement for binding AcO− anions when it is co-bound to Hg2+cations, whereas no affinity for the free receptor by the anion is observed.


 Please wait while we load your content...
Please wait while we load your content...