Phenyl substitution of cationic bis-cyclometalated iridium(iii) complexes for iTMC-LEECs†
Abstract
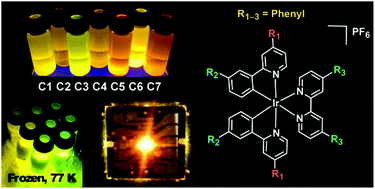
A series of seven cationic bis-cyclometalated iridium(III) complexes of the form [(C^N)2(N^N)Ir][PF6] has been designed in order to examine the effect of bulky, hydrophobic phenyl substituents on the structure–property relationship of these ionic transition metal complexes (iTMCs) in light-emitting electrochemical cells (LEECs). Capping phenyl substituents on the cyclometalating and ancillary ligands allows for individual tuning of the HOMO and LUMO energy levels, respectively, yielding an emission range from yellow to red. The complexes in this series exhibit increased quantum yields, up to 70% higher than the unoptimized, archetypal [(2-phenylpyridine)2(2,2′-bipyridine)Ir][PF6]. Among these, one complex, C3, was recently reported to produce devices with superior luminance and efficiency. Simultaneous measure of the series of complexes enabled the clear discernment of trends in device performance connected to fundamental structure–property relationships that elucidate the origin of enhanced luminance. In general, phenyl substitution of the 2-phenylpyridine ligands of the parent complex produced higher luminance and faster device response than phenyl substitution of the 2,2′-bipyridine ligand. Overall, complex design and device engineering produce competitive LEECs from simple, single-layer architectures. The synthesis, crystallographic, photophysical, and electrochemical properties of the iTMCs, along with the electroluminescence properties of the LEEC devices are reported herein.

 Please wait while we load your content...
Please wait while we load your content...