Structural characterization and proton reduction electrocatalysis of thiolate-bridged bimetallic (CoCo and CoFe) complexes†
Abstract
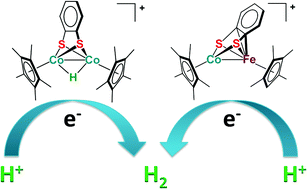
Using an assembly method, dinuclear CoCo and CoFe complexes supported by a bdt ligand, [Cp*Co(μ–η2:η2-bdt)(μ-I)CoCp*][PF6] (1[PF6], Cp* = η5-C5Me5, bdt = benzene-1,2-dithiolate), and [Cp*Co(μ–η2:η4-bdt)FeCp′][PF6] (3[PF6], Cp′ = η5-C5Me4H) were synthesized in high yields. Upon chemical reduction with CoCp2, complexes 1[PF6] and 3[PF6] were converted to [Cp*Co(μ-η2:η2-bdt)CoCp*] (2) and [Cp*Co(μ–η2:η4-bdt)FeCp′][PF6] (3), respectively. Treatment of 2 with HBF4 resulted in the protonation of two cobalt centers to generate a hydride bridged complex, [Cp*Co(μ–η2:η2-bdt)(μ-H)CoCp*][BF4] (4[BF4]), which was identified by spectroscopy and X-ray crystallography. When treating 3 with HBF4, a one-electron oxidation occurred to afford complex 3[BF4] along with the formation of H2. Importantly, heterodinuclear complex 3[PF6] and hydride bridged complex 4[BF4] can serve as effective catalysts to promote proton reduction for hydrogen evolution, as evidenced by cyclic voltammetry.

 Please wait while we load your content...
Please wait while we load your content...