Abstract
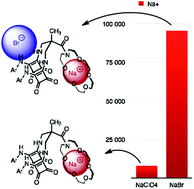
Compounds 2 and 3 were designed and prepared as heteroditopic ion-pair receptors. The design features a 2,2-bis(aminomethyl)propionic acid core to connect and pre-organize binding groups. The cation binding is provided by a sodium selective N-acyl aza-18-crown-6 subunit whereas for anion complexation, two urea groups (receptor 2) or two squaramide groups (receptor 3) were introduced. Beyond acting as anion binding sites, the urea and squaramide groups were used to support sodium cation complexation through metal carbonyl oxygen lone pair interactions. The receptors were found to bind sodium salts of chloride, bromide and nitrate much more strongly than the corresponding ions accompanied by counterions that do not coordinate to the receptor. For example, chloride binding to receptor 2 enhances the strength of sodium complexation by up to 23 times. Conversely, sodium binding enhances chloride recognition by a factor of three. Receptor 3 containing squaramide units, binds sodium chloride and bromide with a similar albeit lower cooperativity. Moreover, unprecedentedly tight binding of these salts was achieved, with association constants as high as log Ka = 6.52 M−1 for NaCl salt complexation.

 Please wait while we load your content...
Please wait while we load your content...