Dinitrogen binding, P4-activation and aza-Büchner ring expansions mediated by an isocyano analogue of the CpCo(CO) fragment†
Abstract
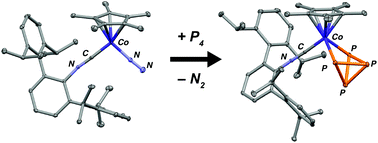
Synthetic studies targeting an m-terphenyl isocyanide analogue of the unstable 16e−, S = 1 complex CpCo(CO) are reported (Cp = η5-C5H5). The m-terphenyl isocyanide CNArDipp2 (ArDipp2 = 2,6-(2,6-(i-Pr)2C6H3)2C6H3) is shown to readily bind to both CpCoI2 and Cp*CoI2 fragments (Cp* = η5-C5Me5) and provide mono-isocyanide starting materials that are suitable for chemical reduction. Treatment of CpCoI2(CNArDipp2) with KC8 produces the bridging isocyanide dimer, [CpCo(μ-CNArDipp2)]2, thereby indicating that the steric combination of Cp and CNArDipp2 ligands does not allow for the production of mononuclear complexes. However, Cp*CoI2(CNArDipp2) with KC8 under an N2 atmosphere results in the formation of the complex, Cp*Co(N2)(CNArDipp2), which is a unique two-legged piano stool complex featuring a coordinated dinitrogen ligand. The N2 ligand in Cp*Co(N2)(CNArDipp2) is shown to be labile and, upon removal by application of vacuum, leads to the production of an η4-coordinated 1-azabenz[b]azulene complex by aza-Büchner cyclization of the CNArDipp2 ligand. This cyclization reaction is rationalized via the intermediacy of the unobserved 16e− species [Cp*Co(CNArDipp2)]. While this intramolecular aza-Büchner cyclization prevents isolation of [Cp*Co(CNArDipp2)], the dinitrogen complex Cp*Co(N2)(CNArDipp2) is shown to serve as a reliable synthon for this 16e− species upon reaction with small molecule substrates. Both free CNArDipp2 and diphenylacetylene react with Cp*Co(N2)(CNArDipp2) to form two-legged piano stool complexes. In addition, Cp*Co(N2)(CNArDipp2) reacts readily with 0.5 and 1.0 equivalents of P4 to produce poly-phosphorus products resulting from P–P single bond cleavage.
- This article is part of the themed collection: Small Molecule Activation

 Please wait while we load your content...
Please wait while we load your content...