Chelating bis-N-heterocyclic carbene complexes of iron(ii) containing bipyridyl ligands as catalyst precursors for oxidation of alcohols†
Abstract
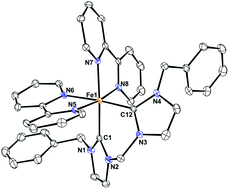
Chelating bis-N-heterocyclic carbene (bis-NHC) complexes of iron(II) containing pyridyl ligands have been prepared by the reaction of [FeCl2L] [L = bipy (1), phen (2)] with [LiN(SiMe3)2] and a bis(imidazolium) salt. The [Fe(bis-NHC)L(I)2] complexes were active pre-catalysts in the oxidation of 1-phenylethanol with tert-butyl hydroperoxide in neat conditions, affording a quantitative yield of acetophenone in 4.5 h. The catalyst could be reused up to six cycles giving a turnover number (TON) of 1500. Various secondary alcohols, both aromatic and aliphatic were selectivity oxidised to the corresponding ketones in excellent yields. Compound 1 is stable in acetonitrile solution for ca. 4 h, although after 16 h, it evolves to a mixture of [Fe(bis-NHC)(bipy)2]I2 (3), [Fe(bipy)3]2+ and bis-imidazolium salt. The molecular structure of 3 has been determined by X-ray diffraction studies.


 Please wait while we load your content...
Please wait while we load your content...