Contrasting coordination behavior of Group 12 perchlorate salts with an acyclic N3O2 donor ligand by X-ray crystallography and 1H NMR†
Abstract
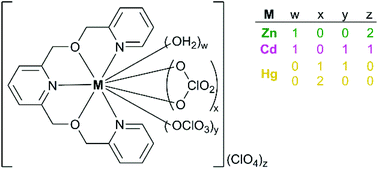
An unbranched N3O2 ligand 2,6-bis[((2-pyridinylmethyl)oxy)methyl]pyridine (L1) was used to prepare new mononuclear heteroleptic Group 12 perchlorate complexes characterized by IR, 1H NMR and X-ray crystallography. Racemic complexes with pentadentate L1 and one to four oxygens from either water or perchlorate bound to a metal ion were structurally characterized. Octahedral [Zn(L1)(OH2)](ClO4)2 (1) and pentagonal bipyramidal [Cd(L1)(OH2)(OClO3)]ClO4 (2) structures were found with lighter congeners. The polymorphic forms of [Hg(L1)(ClO4)2] characterized (3 in P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and 4 in P21/c) had a mix of monodentate, anisobidentate and bidentate perchlorates, providing the first examples of a tricapped trigonal prismatic Hg(II) coordination geometry, as well as additional examples of a rare square antiprismatic Hg(II) coordination geometry. Solution state 1H NMR characterization of the Group 12 complexes in CD3CN indicated intramolecular reorganization remained rapid under conditions where intermolecular M–L1 exchange was slow on the chemical shift time scale for Zn(II) and on the J(M1H) time scale for Cd(II) and Hg(II). Solution studies with more than one equivalent of ligand also suggested that a complex with a 1 : 2 ratio of M : L1 contributed significantly to solution equilibria with Hg(II) but not the other metal ions. The behavior of related linear pentadentate ligands with Group 12 perchlorate salts is discussed.
and 4 in P21/c) had a mix of monodentate, anisobidentate and bidentate perchlorates, providing the first examples of a tricapped trigonal prismatic Hg(II) coordination geometry, as well as additional examples of a rare square antiprismatic Hg(II) coordination geometry. Solution state 1H NMR characterization of the Group 12 complexes in CD3CN indicated intramolecular reorganization remained rapid under conditions where intermolecular M–L1 exchange was slow on the chemical shift time scale for Zn(II) and on the J(M1H) time scale for Cd(II) and Hg(II). Solution studies with more than one equivalent of ligand also suggested that a complex with a 1 : 2 ratio of M : L1 contributed significantly to solution equilibria with Hg(II) but not the other metal ions. The behavior of related linear pentadentate ligands with Group 12 perchlorate salts is discussed.

 Please wait while we load your content...
Please wait while we load your content...