Photoswitchable azobenzene-appended iridium(iii) complexes†
Abstract
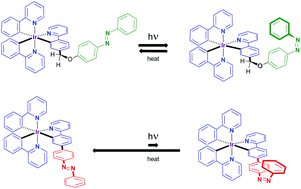
Iridium(III) cyclometalated complexes have been used as models to study the effect that extended conjugation and substitution pattern has on the photochromic behavior of azobenzene-appended 2-phenylpyridyl (ppy) ligands. For this purpose four azobenzene-containing ppy ligands were synthesized. With these ligands, nine iridium(III) complexes containing up to three appended azobenzenes were synthesized. Analysis of their photochromic behaviour by means of UV-vis and 1H-NMR spectroscopy permitted us to conclude that the light-induced trans-to-cis isomerization of the azobenzene was strongly inhibited upon coordination to the Ir(III) cation when the electronic conjugation was extended along the whole ligand. The use of an aliphatic spacer unit (either –CH2– or –OCH2–) between the azobenzene and the ppy fragment of the ligand sufficed to disrupt the electronic communication, and obtain photochromic organometallic complexes.

 Please wait while we load your content...
Please wait while we load your content...