Spotlight on the ligand: luminescent cyclometalated Pt(iv) complexes containing a fluorenyl moiety†
Abstract
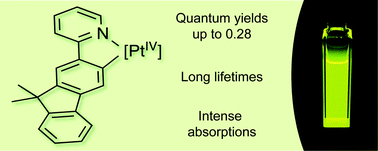
The synthesis, electrochemistry and photophysical properties of a family of Pt(IV) complexes with cyclometalated 2-(9,9-dimethylfluoren-2-yl)pyridine (flpy) are reported. Homoleptic and heteroleptic tris-cyclometalated complexes with a meridional configuration, mer-[Pt(C^N)2(flpy)]OTf, with C^N = flpy or cyclometalated 2-phenylpyridine (ppy), were prepared by reacting the bis-cyclometalated precursors [Pt(C^N)2Cl2] with flpyH in the presence of two equivalents of AgOTf. The corresponding facial isomers were obtained by photoisomerization. The Pt(IV) complexes with flpy display intense absorptions in the near-visible region and yellow phosphorescence in fluid solutions at 298 K, with quantum yields in the range 0.06–0.28 and lifetimes of hundreds of microseconds. The emissions arise from essentially 3LC(flpy) states in all cases, with little metal-orbital contribution. However, computational calculations and experimental data demonstrate that subtle variations in the contribution of metal orbitals to the emitting state have a profound impact on quantum yields, while nonradiative deactivation through the thermal population of deactivating LMCT states does not have a significant influence.


 Please wait while we load your content...
Please wait while we load your content...