Dinuclear Au(i) N-heterocyclic carbene complexes derived from unsymmetrical azolium cyclophane salts: potential probes for live cell imaging applications†
Abstract
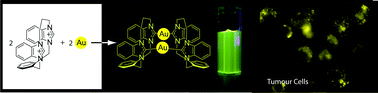
We have synthesized a new series of azolium cyclophanes and used them as precursors of inherently luminescent dinuclear Au(I)–N-heterocyclic carbene (NHC) complexes. The azolium cyclophanes contained two azolium groups (either imidazolium or benzimidazolium), an o-xylyl group, and an alkyl linker chain (either C2, C3 or C4). All of the azolium cyclophanes were characterised by X-ray diffraction studies and VT NMR studies, and all were fluxional in solution on the NMR timescale. The C3- and C4-linked azolium cyclophanes served as precursors of Au2L22+ complexes (L is a cyclophane bis(NHC) ligand). Due to the unsymmetrical nature of the azolium cyclophanes, the Au2L22+ complexes each existed as cis and trans isomers. X-ray diffraction studies showed that the Au2L22+ complexes had short intramolecular Au⋯Au distances, in the range 2.9–3.3 Å, suggestive of an aurophilic attraction, presumably as a consequence of the geometrical constraints imposed by the cyclophane bis(NHC) ligands. The complexes having the shortest Au⋯Au distances (i.e., those based on C3-linked cyclophanes) exhibited intense luminescence in solution. The uptake of one of the dinuclear Au–NHC complexes by tumorigenic cells, and its subsequent distribution and toxicity in the cells, was monitored by luminescence microscopy over 6 h and proliferation measurements, respectively.

 Please wait while we load your content...
Please wait while we load your content...