Novel zirconium complexes with constrained cyclic β-enaminoketonato ligands: improved catalytic capability toward ethylene polymerization†
Abstract
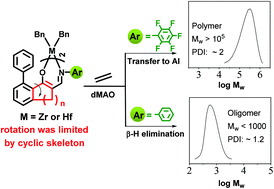
Novel Zr(IV) and Hf(IV) complexes bearing two constrained bulky β-enaminoketonato ligands {[ArN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–C8H3(CH2)n(R)O]2MBn2, M = Zr or Hf; n = 1, 2 or 3; R = H or C6H5; Ar = C6H5 or C6F5} were synthesized and clearly characterized. X-ray crystal structure analysis reveals that these complexes adopt a distorted octahedral geometry. Compared with non-constrained analogues, the Zr(IV) complexes with a cyclic skeleton exhibited high catalytic activities (up to 16.4 kgPE mmolZr−1 h−1) toward ethylene polymerization at ambient pressure and elevated temperatures. Moreover, the catalytic properties of these complexes could be governed exquisitely by appropriate variation of the N-aryl substituents and the size of the benzocyclane. The Zr(IV) complexes bearing a non-fluorinated N-aryl group yielded oligomers, while the fluorinated analogues bearing a five-membered or six-membered cyclane group produced high molecular weight polyethylenes (33.4–306 kg mol−1) under similar conditions on account of the suppression effects on β-H elimination. The Zr(IV) complexes are more active toward ethylene polymerization than the Hf(IV) analogues, and the resulting polymers exhibited higher molecular weight and narrower molecular weight distribution.
CH–C8H3(CH2)n(R)O]2MBn2, M = Zr or Hf; n = 1, 2 or 3; R = H or C6H5; Ar = C6H5 or C6F5} were synthesized and clearly characterized. X-ray crystal structure analysis reveals that these complexes adopt a distorted octahedral geometry. Compared with non-constrained analogues, the Zr(IV) complexes with a cyclic skeleton exhibited high catalytic activities (up to 16.4 kgPE mmolZr−1 h−1) toward ethylene polymerization at ambient pressure and elevated temperatures. Moreover, the catalytic properties of these complexes could be governed exquisitely by appropriate variation of the N-aryl substituents and the size of the benzocyclane. The Zr(IV) complexes bearing a non-fluorinated N-aryl group yielded oligomers, while the fluorinated analogues bearing a five-membered or six-membered cyclane group produced high molecular weight polyethylenes (33.4–306 kg mol−1) under similar conditions on account of the suppression effects on β-H elimination. The Zr(IV) complexes are more active toward ethylene polymerization than the Hf(IV) analogues, and the resulting polymers exhibited higher molecular weight and narrower molecular weight distribution.

 Please wait while we load your content...
Please wait while we load your content...