Suppressing the dendritic growth of zinc in an ionic liquid containing cationic and anionic zinc complexes for battery applications†
Abstract
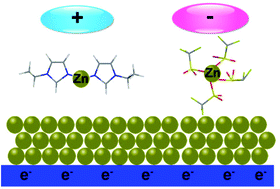
Metallic zinc is a promising negative electrode for high energy rechargeable batteries due to its abundance, low-cost and non-toxic nature. However, the formation of dendritic zinc and low Columbic efficiency in aqueous alkaline solutions during charge/discharge processes remain a great challenge. Here we demonstrate that the dendritic growth of zinc can be effectively suppressed in an ionic liquid electrolyte containing highly concentrated cationic and anionic zinc complexes obtained by dissolving zinc oxide and zinc trifluoromethylsulfonate in a protic ionic liquid, 1-ethylimidazolium trifluoromethylsulfonate. The presence of both cationic and anionic zinc complexes alters the interfacial structure at the electrode/electrolyte interface and influences the nucleation and growth of zinc, leading to compact, homogeneous and dendrite-free zinc coatings. This study also provides insights into the development of highly concentrated metal salts in ionic liquids as electrolytes to deposit dendrite-free zinc as an anode material for energy storage applications.

 Please wait while we load your content...
Please wait while we load your content...