Synthesis, structure, and biological evaluation of a copper(ii) complex with fleroxacin and 1,10-phenanthroline†
Abstract
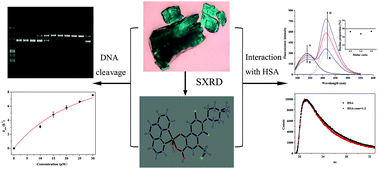
A novel mixed-ligand Cu(II) complex combined with the quinolone drug fleroxacin and 1,10-phenanthroline was synthesized in this work. The crystal structure of the complex was characterized via X-ray crystallography, which was the first reported single crystal complex of fleroxacin. Results showed that Cu(II) was coordinated through pyridone oxygen and one carboxylate oxygen atom of fleroxacin, as well as two nitrogen atoms from 1,10-phenanthroline. Various characterization methods, including Fourier transform infrared, elementary analysis, thermogravimetry, and X-ray powder diffraction, were applied. The Cu(II)–quinolone complex exhibited favorable biological activities, and was proved to be capable of transforming supercoiled PUC19 DNA into nicked form under hydrolytic conditions. The obtained pseudo-Michaelis–Menten kinetic parameter was 12.64 h−1, which corresponded to a million-fold rate enhancement in DNA cleavage. In addition, the interaction capacity of the complex with human serum albumin (HSA) was investigated. The results demonstrated a moderately intense combination between HSA and the complex. The complex evidently quenched the fluorescence of HSA. Approximately 19.2% of the quenching was attributed to Förster resonance energy transfer (FRET), whereas the rest was caused by ground-state complex formation (molar ratio of HSA : complex = 1 : 2). The energy of the complex was excited during FRET, which increased the fluorescence of the complex by approximately 18%.

 Please wait while we load your content...
Please wait while we load your content...