A mechanism for solvent extraction of first row transition metals from chloride media with the ionic liquid tetraoctylammonium oleate
Abstract
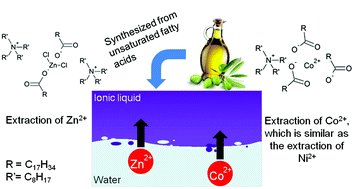
Aqueous waste streams of the metallurgical industry often contain considerable concentrations of metal salts. Previous research showed that the metal chloride salts of zinc(II), manganese(II) and iron(III) can be recovered by solvent extraction using a sustainable and renewable fatty acid based ionic liquid as the extractant. In this paper, the extraction mechanism of Zn(II), Co(II) and Ni(II) from chloride media has been studied systematically. The metal extraction performances of the precursors, sodium oleate and tetraoctylammonium chloride, were compared to the extraction performance of the ionic liquid tetraoctylammonium oleate. Slope analysis experiments were performed to determine the number of ionic liquid molecules involved in the extraction. The experimental data showed that Co(II) and Ni(II) were extracted in the pH range from 6 to 8 by the formation of negatively charged metal carboxylate complexes with tetraalkylammonium counter ions. In contrast, Zn(II) gets extracted as a mixed metal chloride carboxylate anionic complex with tetraalkylammonium counter ions. This extraction mechanism was supported by EXAFS measurements.

 Please wait while we load your content...
Please wait while we load your content...