Titanium oxo-clusters derivatized from the Ti10O12(cat)8(py)8 complex: structural investigation and spectroscopic studies of light absorption†
Abstract
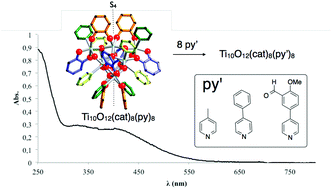
A series of deep-red colored nano-sized titanium oxo-clusters bearing catecholato ligands is reported. These architectures are produced via post-synthetic modification of the Ti10O12(cat)8(py)8 (cat = catecholato, py = pyridine) complex through quantitative substitution of labile pyridine ligands by three substituted pyridines (pico, 4-Phpy and pyrald). The crystal structure analysis reveals a common Ti10O12(cat)8 backbone for the three isolated molecular architectures. Partial charge analysis indicates two types of titanium atoms within these complexes with one resembling titanium(IV) found in TiO2. These complexes strongly absorb visible light in solution (λmax = 411 nm, ε = 10 800 for Ti10O12(cat)8(py)8 in CHCl3) and in the solid-state. The band gaps estimated from the reflectance spectra are between 1.85 eV and 1.97 eV. The present work also details the HOMO and LUMO representations obtained via DFT calculation for Ti10O12(cat)8(py)8 and a virtual Ti10O12(cat)8 complex as well as the DOS (density of states) plots calculated for those structures. This computational study highlights an impact of the pyridine ligand on the DOS plots.

 Please wait while we load your content...
Please wait while we load your content...