Electrochemical and chemical routes to hydride loss from an iridium dihydride†
Abstract
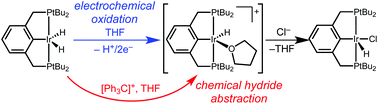
With a view towards replacing sacrificial hydrogen acceptors in alkane dehydrogenation catalysis, electrochemical methods for oxidative activation of a pincer-ligated iridium hydride intermediate were explored. A 1H+/2e− oxidation process was observed in THF solvent, with net hydride loss leading to a reactive cationic intermediate that can be trapped by chloride. Analogous reactivity was observed with the concerted hydride transfer reagent Ph3C+, connecting chemical and electrochemical hydride loss pathways.
- This article is part of the themed collection: New Talent: Americas

 Please wait while we load your content...
Please wait while we load your content...