Probing the electrochemical properties of biopolymer modified EMD nanoflakes through electrodeposition for high performance alkaline batteries†
Abstract
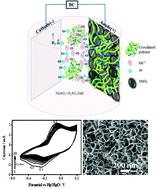
In the present work, a novel biopolymer approach has been made to electrodeposit manganese dioxide from manganese sulphate in a sulphuric acid bath containing chitosan in the absence and presence of glutaraldehyde as a cross-linking agent. Galvanostatically synthesised electrolytic manganese dioxide (EMD) nanoflakes were used as electrode materials and their electrochemical properties with the influence of biopolymer chitosan were systematically characterized. The structural determination, surface morphology and porosity of nanostructured EMD were evaluated using X-ray diffraction, Fourier transform infrared spectroscopy, field emission scanning electron microscopy and nitrogen adsorption–desorption techniques. The results obtained were compared with that of blank EMD (polymer free). The results indicated that the EMD having chitosan cross-linked with glutaraldehyde possesses a reduced particle size and more porous structure than the blank and EMDs synthesized in the presence of chitosan but without glutaraldehyde. The results revealed that chitosan was unable to play any significant role on its own but chitosan in the presence of glutaraldehyde forms a cross-linking structure, which in turn influences the nucleation and growth of the EMDs during electrodeposition. EMDs obtained in the presence of chitosan (1 g dm−3) and glutaraldehyde (1% glutaraldehyde) exhibited a reversible and better discharge capacity upon cycling than the blank which showed its typical capacity fading behaviour with cycling. In addition, EMD synthesized in the presence of 1 g dm−3 chitosan and 2% glutaraldehyde exhibited a superior electrochemical performance than the blank and lower amounts (1%; 1.5%) of glutaraldehyde, showing a stable discharge capacity of 60 mA h g−1 recorded up to 40 cycles in alkaline KOH electrolyte for a Zn–MnO2 system. Our results demonstrate the potential of using polymer modified EMDs as a new generation of alkaline battery materials. The XPS data show that a surface functional moiety arising from the cross-linked chitosan enhances the electrochemical properties of the Zn–MnO2 system.

 Please wait while we load your content...
Please wait while we load your content...