Triphos derivatives and diphosphines as ligands in the ruthenium-catalysed alcohol amination with NH3†
Abstract
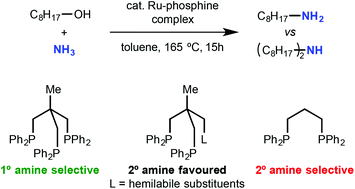
The ruthenium-triphos and diphosphine-catalysed amination of alcohols with ammonia is reported. Various types of triphos derivatives with electron-donating functional group were synthesized and used as ligands in the Ru-catalysed alcohol amination with NH3. The triphos derivatives are effective for the formation of primary amines. On the other hand, if hemilabile diphosphines as tridentate ligands are used, mixtures of secondary-along with primary amines are obtained. It was found that even simple diphosphines can be used as ligands for the selective formation of the secondary amines. The diphosphine system allows a new entry to the Ru-catalysed formation of secondary amines.

 Please wait while we load your content...
Please wait while we load your content...