New amide–chloride phases in the Li–Al–N–H–Cl system: formation and hydrogen storage behaviour†
Abstract
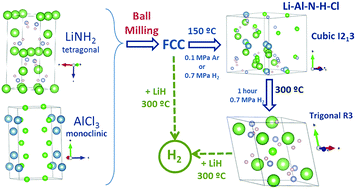
New amide–chloride phases were successfully synthesized by mechanical milling of the LiNH2–AlCl3 mixture at a molar ratio of 1 : 0.11 and further heating at 150 °C under argon (0.1 MPa) or under hydrogen pressure (0.7 MPa). Powder X-ray diffraction measurements as a function of milling time increase revealed that the milling of the LiNH2–0.11AlCl3 mixture results in the formation of a FCC solid solution with an excess of LiNH2. Subsequent heating of the LiNH2–0.11AlCl3 sample ball milled for 5 hours at 150 °C under argon or under hydrogen induces the appearance of an amide–chloride phase isostructural with cubic Li4(NH2)3Cl. This Li–Al–N–H–Cl phase transforms progressively into the trigonal phase after prolonged heating at 300 °C under hydrogen pressure. The thermal behaviour of the amide–chloride without and with LiH addition displays dissimilar decomposition pathways. The decomposition of amide–chloride alone involves the formation of ammonia and hydrogen from 120 to 300 °C. Conversely, the amide–chloride material in the presence of LiH only releases hydrogen avoiding the emission of ammonia. The resultant material is able to be rehydrogenated under moderate conditions (300 °C, 0.7 MPa H2), providing a new reversible hydrogen storage system.

 Please wait while we load your content...
Please wait while we load your content...