Spectroscopic investigations into the binding of hydrogen sulfide to synthetic picket-fence porphyrins†
Abstract
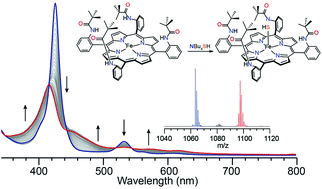
The reversible binding of hydrogen sulfide (H2S) to hemeprotein sites has been attributed to several factors, likely working in concert, including the protected binding pocket environment, proximal hydrogen bond interactions, and iron ligation environment. To investigate the importance of a sterically-constrained, protected environment on sulfide reactivity with heme centers, we report here the reactivity of H2S and HS− with the picket-fence porphyrin system. Our results indicate that the picket-fence porphyrin does not bind H2S in the ferric or ferrous state. By contrast, reaction of the ferric scaffold with HS− results in reduction to the ferrous species, followed by ligation of one equivalent of HS−, as evidenced by UV-vis, NMR spectroscopy and mass spectrometry studies. Measurement of the HS− binding affinities in the picket-fence or tetraphenyl porphyrin systems revealed identical binding. Taken together, these results suggest that the protected, sterically-constrained binding pocket alone is not the primary contributor for stabilization of ferric H2S/HS− species in model systems, but that other interactions, such as hydrogen bonding, must play a critical role in facilitation of reversible interactions in ferric hemes.

 Please wait while we load your content...
Please wait while we load your content...