The design of second generation MOP-phosphonites: efficient chiral hydrosilylation of functionalised styrenes†
Abstract
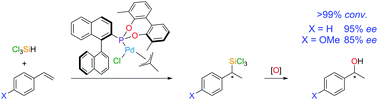
A series of enantiopure MOP-phosphonite ligands, with tailored steric profiles, have been synthesised and are proven to be very successful in high-yielding, regio- and enantioselective catalytic hydrosilylation reactions of substituted styrenes, affording important chiral secondary alcohols.
- This article is part of the themed collection: Phosphorus Chemistry: Discoveries and Advances

 Please wait while we load your content...
Please wait while we load your content...