Ruthenium olefin metathesis catalysts featuring unsymmetrical N-heterocyclic carbenes†
Abstract
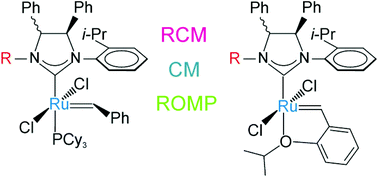
New ruthenium Grubbs’ and Hoveyda–Grubbs’ second generation catalysts bearing N-alkyl/N-isopropylphenyl N-heterocyclic carbene (NHC) ligands with syn or anti backbone configuration were obtained and compared in model olefin metathesis reactions. Different catalytic efficiencies were observed depending on the size of the N-alkyl group (methyl or cyclohexyl) and on the backbone configuration. The presence of an N-cyclohexyl substituent determined the most significant reactivity differences between catalysts with syn or anti phenyl groups on the backbone. In particular, anti catalysts proved highly efficient, especially in the ring-closing metathesis (RCM) of encumbered diolefins, while syn catalysts showed low efficiency in the RCM of less hindered diolefins. This peculiar behavior, rationalized through DFT studies, was found to be related to the high propensity of these catalysts to give nonproductive metathesis events. Enantiopure anti catalysts were also tested in asymmetric metathesis reactions, where moderate enantioselectivities were observed. The steric and electronic properties of unsymmetrical NHCs with the N-cyclohexyl group were then evaluated using the corresponding rhodium complexes. While steric factors proved unimportant for both syn and anti NHCs, a major electron-donating character was found for the unsymmetrical NHC with anti phenyl substituents on the backbone.

 Please wait while we load your content...
Please wait while we load your content...