Theoretical study of the mechanism for the sequential N–O and N–N bond cleavage within N2O adducts of N-heterocyclic carbenes by a vanadium(iii) complex†
Abstract
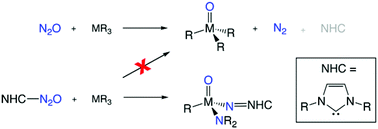
A theoretical study into the reactions of the N2O adducts of N-heterocyclic carbenes (NHCs) and a V(III) complex was carried out using DFT calculations. Unlike most transition metal reactions with N2O that simply release N2 following O-atom transfer onto the metal centre, this NHC-based system traps the entire N2O molecule and then cleaves both the N–O and N–N bond in two consecutive reactions. The NHC presence increases the reactivity of N2O by altering the distribution of electron density away from the O-atom towards the two N-atoms. This electronic redistribution enables V–N binding interactions to form a reactive N,O-donor intermediate species. Our results show that bond breaking with concomitant ligand migration occurs via a concerted process for both the N–O and N–N cleavage reactions.

 Please wait while we load your content...
Please wait while we load your content...