Performances and mechanistic investigations of a triphosphine trioxide/ionic liquid system for rare earth extraction†
Abstract
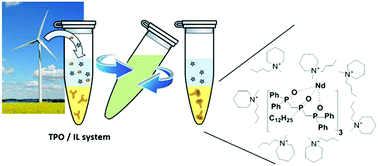
The extraction of rare earth elements (REEs) from nitric acid solution with a triphosphine trioxide (TPO) is presented. Performances of such a ligand in ionic liquids vs. a classical solvent (benzyl ether) are compared. TPO seems to be 10 to 100 times more efficient when it is dissolved in ionic media whatever the concentration of nitric acid involved. Mechanistic investigations reveal that cation exchange classically observed in ionic liquids is not consistent with the experimental data. Moreover, clear differences in the TPO/Ln complexes between classical and ionic media are highlighted. A stable complex of 1 lanthanide for 3 TPO is formed in an ionic liquid whereas a complex of 1 lanthanide for 6 to 9 TPO is formed in benzyl ether. Back extraction is also studied and good recovery of REEs could be obtained. The TPO/ionic liquid system shows remarkable performances i.e. efficiency and selectivity towards lanthanides in a simulated leaching solution of a Nd/Fe/B/Dy magnet.

 Please wait while we load your content...
Please wait while we load your content...