Tuning the Mn valence state in new Ca0.66Mn2−xAlxO4 (x ≤ 0.4) oxides: impact on magnetic and redox properties†
Abstract
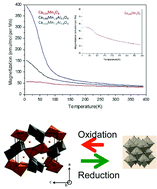
New Ca0.66Mn2−xAlxO4 (x ≤ 0.4) solid solutions crystallizing with the CaFe2O4-type structure (SG: Pnma) were synthesized for the first time by the glycine-nitrate process. The structures were determined on the basis of XRD Rietveld analysis and electron diffraction investigation. While the CaMn3O6 (‘Ca0.66Mn2O4’) oxide adopts a monoclinic unit cell, the Al substitution for Mn (x = 0.2, 0.4) leads to an orthorhombic cell with only two Mn atomic positions, with different valence states, and 33% of Ca sites empty. The Ca molar concentration decreases down to 0.6 in order to increase the Mn valence leading to a phase mixture, whereas a slight Ca content increase up to 0.7 leads to a decrease of Mn valence in the pure phase. The Al3+ ions are located at a specific Mn site because their ionic radii are close to that of Mn4+ and a more isotropic environment. The unit cell parameters and volume strongly decrease for a low Al content and tend to an asymptotic value of x = 0.33–0.4, around the limit of solubility. As the Al content increases, the Mn valence state in the same slightly distorted octahedral site increases up to 4+ whereas the other octahedral site is highly elongated and corresponds mainly to Jahn–Teller Mn3+. At x = 0.33, these two Mn sites correspond to Mn4+ and Mn3+ respectively. Moreover, the aluminium content increase induces a weakening of the global antiferromagnetic long range interactions between the ferromagnetic chains. The Al substitution leads to the change of the Mn valence distribution as well as the unit cell symmetry of the CaMn3O6 phase. These 1D tunnel networks stabilizing the Mn3+/Mn4+ valence states can be reduced under Ar/5%H2 between T = 300 °C and T = 600 °C (heating rate = 2 °C min−1) into pure Mn2+ rocksalt solid solution despite the large difference in ionic radii. The re-oxidation leads to the same CaFe2O4-type structure and several redox cycles can be operated. The relationship between the two double chains of the edge-sharing octahedral sites and the rocksalt-type framework is clear and should appear as the driving force for the structural transformation during the reduction/oxidation processes. Finally, Al substitution allows an increasing of the Mn–O bond covalence and consequently the reduction in temperature.

 Please wait while we load your content...
Please wait while we load your content...