Cytotoxicity and preliminary mode of action studies of novel 2-aryl-4-thiopyrone-based organometallics†
Abstract
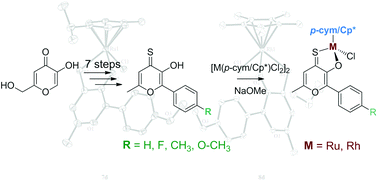
Organometallic complexes with thiopyrone-based ligands have shown promising cytotoxic activity in vitro. To investigate the impact of the ligand backbone modification of these biologically active compounds and enhance the solubility in aqueous solution, the (thio)pyrone scaffold was modified via Suzuki–Miyaura coupling reaction and converted into corresponding organometallic Ru(II) and Rh(III) complexes. Characterization of the synthesized compounds was carried out by means of 1D and 2D NMR, ESI MS, and also by X-ray diffraction analysis. The stability in aqueous solution was investigated via1H NMR spectroscopy. Due to the close structural resemblance to flavonoids, topoisomerase inhibition, the cytotoxicity in human cancer cell lines as well as ROS generation was investigated by means of the topoisomerase II drug screening assay, the MTT assay and DCFH-DA assay, respectively.

 Please wait while we load your content...
Please wait while we load your content...