Size-selective electrocatalytic activity of (Pt)n/MoS2 for oxygen reduction reaction†
Abstract
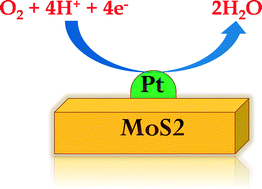
In the present work, we have investigated the electrocatalytic activity of the oxygen reduction reaction (ORR), O2 + 4H+ + 4e− → 2H2O, for (Pt)n clusters (n = 1, 2, 3, 5, 7, 10 and 12) adsorbed on semiconducting (2H) and metallic (1T) MoS2 monolayers using first principles density functional theory. We have considered four elementary reactions involved in ORR within a unified electrochemical thermodynamic framework and the corresponding Gibbs adsorption free energies of the key intermediates (*OOH, *O, *OH) associated with each step have been calculated. The results indicate that the reduction of adsorbed hydroxyl (*OH) to water (*OH + H+ + e− → H2O) is the bottleneck step in the ORR process. The adsorption free energy of *OH (ΔG*OH) is found to be the thermodynamic descriptor for the present systems. Eventually, the ORR activity has been described as a function of ΔG*OH and a volcano plot predicting (Pt)7/2H-MoS2 as the best ORR catalyst amongst the (Pt)n/MoS2 heterosystems with an overpotential value of 0.33 V has been established. Our finding proposes a new promising electrocatalyst towards better activity for ORR with very small amount of Pt loading.

 Please wait while we load your content...
Please wait while we load your content...