Catalytic activity of Pt38 in the oxygen reduction reaction from first-principles simulations
Abstract
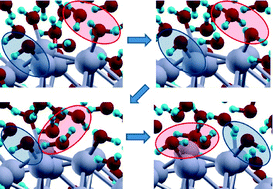
The activity of truncated octahedral Pt38 clusters as a catalyst in the oxygen reduction reaction (ORR) is investigated via first-principles simulations. Three catalytic steps: O2 dissociation (O2ads → 2Oads), O hydration (Oads + H2Oads → 2OHads), and H2O formation (OHads + Hads → H2Oads) are considered, in which all reactant species are co-adsorbed on the Pt38 cluster according to a Langmuir–Hinshelwood mechanism. The minimum structures and saddle points for these different steps are then calculated at the density-functional theory (DFT) level using a gradient-corrected exchange–correlation (xc-)functional and taking into account the effect of the solvent via a self-consistent continuum solvation model. Moreover, first-principles molecular dynamics (AIMD) simulations in which the H2O solvent is explicitly described are performed to explore dynamic phenomena such as fast hydrogen transfer via meta-stable hydronium-type configurations and their possible role in ORR reaction paths. By comparing the present findings with previous results on the Pt(111) surface, it is shown that in such a nanometer-size cluster the rate-determining-step (rds) corresponds to H2O formation, at variance with the extended surface in which O hydration was rate-determining, and that the overall reaction barrier is actually increased with respect to the extended system. This is in agreement with and rationalizes experimental results showing a decrease of ORR catalytic activity in the nanometer-size cluster range.
- This article is part of the themed collection: Nanocatalysis

 Please wait while we load your content...
Please wait while we load your content...