Theoretical investigations of non-noble metal single-atom catalysis: Ni1/FeOx for CO oxidation
Abstract
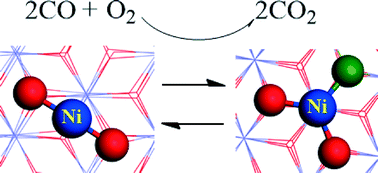
Significant progress has recently been made in single-atom catalysis involving noble metals. We report here a theoretical investigation of the catalytic mechanism of CO oxidation of a non-noble metal single-atom catalyst (SAC) Ni1/FeOx using density functional theory (DFT). The calculated results show that this new SAC Ni1/FeOx has a high catalytic activity at room temperature for CO oxidation. CO adsorption strength is a key factor in determining catalytic activity for CO oxidation. Compared with noble-metal catalysts Pt1/FeOx and Ir1/FeOx, the catalytic activity for CO oxidation of Ni1/FeOx is found to be comparable to that of Pt1/FeOx, but is considerably higher than that of Ir1/FeOx. Our theoretical prediction of this new non-noble metal Ni1/FeOx catalyst with high catalytic activity for CO oxidation at room temperature may find practical applications. The theoretical investigation provides a fundamental understanding of the catalytic mechanism of singly-dispersed surface atoms and helps to stimulate further experimental studies on highly active non-noble metal single-atom catalysts.
- This article is part of the themed collection: Nanocatalysis

 Please wait while we load your content...
Please wait while we load your content...